Des preuves que l’usine de fabrication d’ARNm de Pfizer à Andover était loin d’être conforme aux normes de fabrication
- Obtenir le lien
- X
- Autres applications
De : https://hedleyrees.substack.com/p/rock-solid-evidence-that-pfizers?
TRADUCTION AUTOMATIQUE : https://translate.google.com/website?sl=hl&en=fr&hl=fr&u=https://lezarceleurs.blogspot.com/2024/07/des-preuves-que-lusine-de-fabrication.html
Pfizer should never have been allowed to put its products into patient's bodies
The Rock Solid Evidence provided by FDA
As subscribers may know, I have been in communication with the FDA’s Center for Biologics Evaluation and Review on the topic of facility inspections that unearthed deep manufacturing issues in facilities producing the SARS-CoV-2 injections drug substance (DS) and drug product (DP). This relates to Pfizer/BioNTech and Moderna.
FDA has been incredibly cooperative, and that is the way I have known them to be ever since me and my company, PharmaFlow, began working with them in 2010.
Yes, through this whole debacle, there has been interference at the top, but the inspectors in the field have still been doing their highly important work identifying issues.
This is what I’ve found out, beginning with a response to my FOIA:
“Dear Mr. Rees,
Thank you for speaking with me earlier today about your Freedom of Information Act (FOIA) request # 2024-5142. Please find attached the FOIA response from the Center for Biologics Evaluation and Research (CBER).
For your convenience, I did also find this document web posted via our CBER eFOI reading room and the file sharing platform box.com (https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/biologics-electronic-reading-room-efoia) and I believe it can be directly accessed here: https://fdahhs.ent.box.com/s/xaaal1mghm0wxl56iqeenyk2t396zueh/file/1525382845444
Sincerely,
Elizabeth Sly, M.S.
Branch Chief, ALFOIB
Phone: 240.402.8001
Email: Elizabeth.Sly@fda.hhs.gov
Access Litigation and Freedom of Information Branch (ALFOIB)
Division of Disclosure and Oversight Management (DDOM)
Office of Communication Outreach and Development (OCOD)
Center for Biologics Evaluation and Research (CBER)
U.S. Food and Drug Administration (FDA)
This is a screenshot of the response letter:

This is a screenshot of the first page of the Establishment Inspection Report (EIR):
It states that a previous inspection in April/May 2019 resulted in the issuance of a three-item Form [FDA] 483.
What is an FDA Form 483? Here are frequently asked questions. The first two Q/As are:
Q: When is an FDA Form 483 issued?
A: An FDA Form 483 is issued to firm management at the conclusion of an inspection when an investigator(s) has observed any conditions that in their judgment may constitute violations of the Food Drug and Cosmetic (FD&C) Act and related Acts. FDA investigators are trained to ensure that each observation noted on the FDA Form 483 is clear, specific and significant. Observations are made when in the investigator’s judgment, conditions or practices observed would indicate that any food, drug, device or cosmetic has been adulterated or is being prepared, packed, or held under conditions whereby it may become adulterated or rendered injurious to health.
Q: What is the purpose of an FDA Form 483?
A: The FDA Form 483 notifies the company’s management of objectionable conditions. At the conclusion of an inspection, the FDA Form 483 is presented and discussed with the company’s senior management. Companies are encouraged to respond to the FDA Form 483 in writing with their corrective action plan and then implement that corrective action plan expeditiously.
If you think that’s not good news, wait until you hear this:
This is the Establishment Inspection Report that FDA provided:
It is 86 pages, so you may not have time or inclination to read it all, so I’ll pick out the key points.
The Signature Page at the end identifies the inspectors and their commitment to their findings:
Kathleen R. Jones, Biologist, CBER/OCBQ/DMPQ/MRB1
Ekaterina Allen, CSO, CBER/OCBQ/DMPQ/MRB2
Anissa Cheung, CSO, CBER/OVRR/DVP
Debra M. Emerson, CSO, Team Biologics
These are screenshots of discussions with management following the inspections. Some of it is redacted, but there is enough in there to indicate how bad things were, eg:
“Operators were observed kneeling on the floor and reaching under a cart to plug into an electrical outlet, placing MBRs (Batch Manufacturing Records) and printed SOPs (Standard Operating Procedures) and pens on top of SUMs being used in manufacture.”
More like that and worse below:

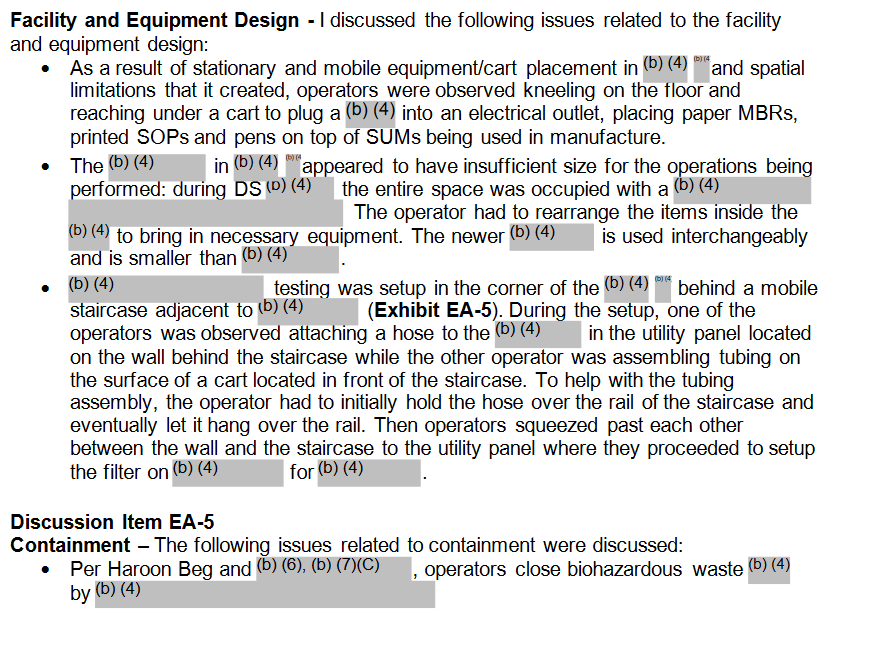
This would have been passed up the FDA chain of command
There can be no doubt that this will have been passed up the chain of command at FDA.
Did it land on the desk of Peter Marks M.D., PhD., Director - Center for Biologics Evaluation and Research (CBER).
The same Peter Marks that is pushing ahead with gene therapy for rare disease:
With Duchenne decision ahead, FDA’s Marks pushes for speedy gene therapy approvals
“In rare diseases, we need something now, not later,” said Peter Marks, head of the FDA office that reviews gene therapies, at a conference held Friday.
Peter Doshi, Senior Editor at the BMJ, has this evidence
Peter Doshi, Senior Editor at the BMJ, is in possession of this evidence, following this post below:
Hopefully, he will find it useful.
Will be back with more soon.
Regards, Hedley
- Obtenir le lien
- X
- Autres applications





Commentaires
Enregistrer un commentaire